


| alpha-(Aminomethyl)-4-hydroxybenzyl alcohol hydrochloride |
| (+/-)-1-(4-Hydroxyphenyl)-2-aminoethanol hydrochloride |
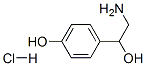
※ 화학명 : Octopamine HCl, 98%
※ 등급 : APIs and Bioactives > Adrenergic Agonists
※ 판매 포장단위 : 1g , 5g , 10g , 25g
※ 화학식 : C8H12ClNO2
※ CAS # : [770-05-8]
|
| IDENTITY |
|
| CAS Registry #: |
[770-05-8] |
| MDL Number: |
MFCD00012881 |
| MF: |
C8H12ClNO2 |
| MW: |
189.64 | |
| SPECIFICATIONS & PROPERTIES |
|
| Purity: |
98% |
| Physical Form: |
Crystals |
| Melting Point: |
169-171°C |
| Long-Term Storage: |
Store at room temperature |
| Shipping: |
Not Regulated for Shipping | | |
BIOLOGICAL INFO |
|
| Form: |
HCl salt |
REVIEWOctopamine (beta, 4-dihydroxyphenethylamine) is an endogenous biogenic amine that is closely related to norepinephrine, and has effects on the adrenergic and dopaminergic systems. Octopamine HCl, a racemate, is an alpha-adrenergic sympathomimetic amine, biosynthesized from tyramine in the CNS and platelets and also in invertebrate nervous systems. It is used to treat hypotension and as a cardiotonic. The natural D(-) form is more potent than the L(+) form in producing cardiovascular adrenergic responses.. Under the trade names Epirenor, Norden, and Norfen, octopamine is also used clinically as a sympathomimetic agent. It is also a neurotransmitter in some invertebrates.
REFERENCES
[1] |
Mannich, C.; Thiele, E. beta-Hydroxy-beta-phenylethylamine and related compounds. Archiv der Pharmazie (Weinheim, Germany) (1915), 253, 181-95. |
| [2] |
Corrigan, John R.; Langerman, Marie-Jo; Moore, Maurice L. N-Substituted 1-(p-hydroxyphenyl)-2-aminoethanols. Journal of the American Chemical Society (1945), 67, 1894-6. |
| [3] |
Fujiwara, Motohatsu; Hattori, Keisuke; Mizusawa, Hideo; Muryobayashi, Takashi; Kato, Yoshiko Pharmacological action octopamine, with special reference to biochemical conversion to noradrenaline. Japanese Journal of Pharmacology (1968), 18(1), 113-29. |
| [4] |
Jagiello-W?jtowicz E. Mechanism of central action of octopamine. Pol J Pharmacol Pharm. 1979 Sep-Oct;31(5):509-16. |
Risk Description(s)
| R36/37/38: |
Irritating to eyes, respiratory system and skin |
Safety Description(s)
| S24/25: |
Avoid contact with skin and eyes. |



고액결제의 경우 안전을 위해 카드사에서 확인전화를 드릴 수도 있습니다. 확인과정에서 도난 카드의 사용이나 타인 명의의 주문등 정상적인 주문이 아니라고 판단될 경우 임의로 주문을 보류 또는 취소할 수 있습니다.
무통장 입금은 상품 구매 대금은 PC뱅킹, 인터넷뱅킹, 텔레뱅킹 혹은 가까운 은행에서 직접 입금하시면 됩니다.
주문시 입력한 입금자명과 실제입금자의 성명이 반드시 일치하여야 하며, 7일 이내로 입금을 하셔야 하며 입금되지 않은 주문은 자동취소 됩니다.
배송 방법 : 택배 및 직납
배송 지역 : 전국
배송 비용 : 총 구매 비용이 ₩100,000 (VAT별도) 미만일 경우 착불로 발송
배송 기간 : 국산품 (3~7일), 수입품 (7~14일)
배송 안내
- 산간벽지나 도서지방은 별도의 추가금액을 지불하셔야 하는 경우가 있습니다.
고객님께서 주문하신 상품은 입금 확인후 배송해 드립니다. 다만, 상품종류에 따라서 상품의 배송이 다소 지연될 수 있습니다.



교환 및 반품이 가능한 경우
- 상품을 공급 받으신 날로부터 7일이내
교환 및 반품이 불가능한 경우
- 고객님의 책임 있는 사유로 상품등이 멸실 또는 훼손된 경우
- 포장을 개봉하였거나 포장이 훼손되어 상품가치가 상실된 경우
- 시간의 경과에 의하여 재판매가 곤란할 정도로 상품등의 가치가 현저히 감소한 경우
- 온도관리품 및 수입품은 반품이 불가능합니다.
※ 주문 취소 및 반품으로 환불을 요청하실 경우에는 E-mail(info@syinnovation.co.kr) 상담서비스나 고객만족센터(070-4829-4101)를 통해 요청하시면 친절하게 처리해 드리겠습니다.











